LIGHT REACTION
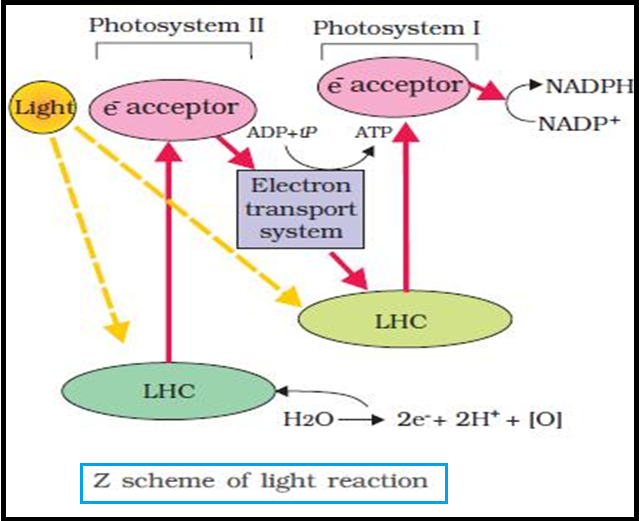
● `color{brown}"Light reactions"` or the `color{Brown}"Photochemical phase"` include `color{violet}"light absorption"`, `color{violet}"water splitting"`, `color{violet}"oxygen release"`, and the formation of `color{violet}"high-energy chemical intermediates,"` ATP and NADPH.
● `color{violet}"Several complexes"` are involved in the process.
● The `color{violet}"pigments"` are organised into two discrete `color{violet}"photochemical light"` `color{violet}"harvesting complexes (LHC)"` within the `color{Brown}"Photosystem I (PS I)"` and `color{Brown}"Photosystem II (PS II)"`.
● These are named in the `color{violet}"sequence of their discovery"`, and not in the `color{violet}"sequence in which they function"` during the light reaction.
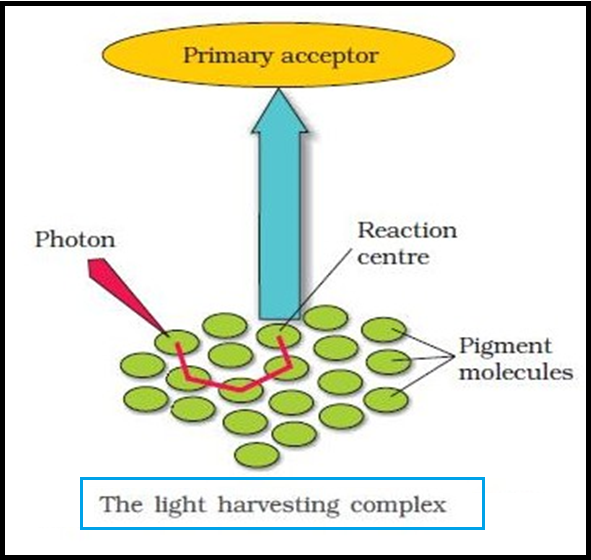
● The `color{Brown}"LHC"` are made up of `color{violet}"hundreds"` of `color{violet}"pigment molecules"` bound to `color{violet}"proteins"`.
● `color{violet}"Each photosystem"` has all the `color{violet}"pigments"` (except `color{violet}"one molecule of chlorophyll a"`) forming a `color{violet}"light harvesting system"` also called `color{Brown}"antennae"`.
● These pigments help to make `color{violet}"photosynthesis more efficient"` by `color{violet}"absorbing different wavelengths"` of light.
● The `color{violet}"single chlorophyll a"` molecule forms the `color{brown}"reaction centre"`.
● The `color{violet}"reaction centre"` is `color{violet}"different"` in both the photosystems.
● In `color{Brown}"PS I"` the reaction centre chlorophyll a has an `color{violet}"absorption peak at 700 nm"`, hence is called `color{Brown}"P700"`, while in `color{violet}"PS II"` it has `color{violet}"absorption maxima at 680 nm"`, and is called `color{Brown}"P680"`.
● `color{violet}"Several complexes"` are involved in the process.
● The `color{violet}"pigments"` are organised into two discrete `color{violet}"photochemical light"` `color{violet}"harvesting complexes (LHC)"` within the `color{Brown}"Photosystem I (PS I)"` and `color{Brown}"Photosystem II (PS II)"`.
● These are named in the `color{violet}"sequence of their discovery"`, and not in the `color{violet}"sequence in which they function"` during the light reaction.
● The `color{Brown}"LHC"` are made up of `color{violet}"hundreds"` of `color{violet}"pigment molecules"` bound to `color{violet}"proteins"`.
● `color{violet}"Each photosystem"` has all the `color{violet}"pigments"` (except `color{violet}"one molecule of chlorophyll a"`) forming a `color{violet}"light harvesting system"` also called `color{Brown}"antennae"`.
● These pigments help to make `color{violet}"photosynthesis more efficient"` by `color{violet}"absorbing different wavelengths"` of light.
● The `color{violet}"single chlorophyll a"` molecule forms the `color{brown}"reaction centre"`.
● The `color{violet}"reaction centre"` is `color{violet}"different"` in both the photosystems.
● In `color{Brown}"PS I"` the reaction centre chlorophyll a has an `color{violet}"absorption peak at 700 nm"`, hence is called `color{Brown}"P700"`, while in `color{violet}"PS II"` it has `color{violet}"absorption maxima at 680 nm"`, and is called `color{Brown}"P680"`.